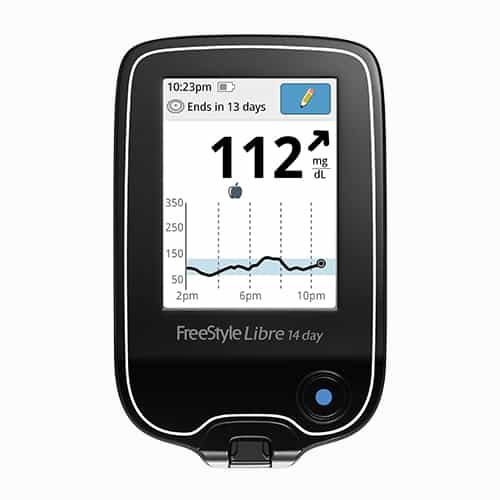
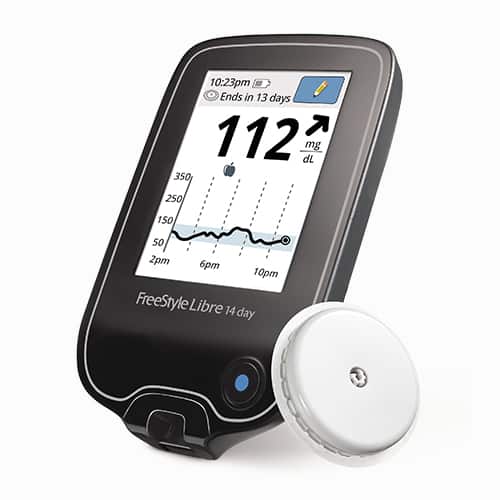
FreeStyle Libre and FreeStyle Libre 14 day Flash Glucose Monitoring systems are continuous glucose monitoring (CGM) devices indicated for replacing blood glucose testing and detecting trends and tracking patterns aiding in the detection of episodes of hyperglycemia and hypoglycemia, facilitating both acute and long-term therapy adjustments in persons with diabetes. The systems are intended for single patient use and require a prescription.
CONTRAINDICATIONS: Remove the sensor before MRI, CT scan, X-ray, or diathermy treatment.
WARNINGS/LIMITATIONS: Do not ignore symptoms that may be due to low or high blood glucose, hypoglycemic unawareness, or dehydration. Check sensor glucose readings with a blood glucose meter when Check Blood Glucose symbol appears, when symptoms do not match system readings, or when readings are suspected to be inaccurate. The systems do not have alarms unless the sensor is scanned, and the systems contain small parts that may be dangerous if swallowed. The systems are not approved for pregnant women, persons on dialysis, or critically-ill population. Sensor placement is not approved for sites other than the back of the arm and standard precautions for transmission of blood borne pathogens should be taken. The built-in blood glucose meter is not for use on dehydrated, hypotensive, in shock, hyperglycemic-hyperosmolar state, with or without ketosis, neonates, critically-ill patients, or for diagnosis or screening of diabetes. When using FreeStyle LibreLink app, access to a blood glucose monitoring system is required as the app does not provide one.